Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
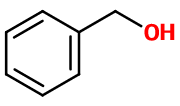
Alcool Benzylique - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 100-51-6
-
EINECS number : 202-859-9
-
FEMA number : 2137
-
FLAVIS number : 02.010
-
JECFA number : 25
-
Appearance : Colorless liquid
-
Density : 1,04
-
Volatility : Heart/Base
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C7H8O
-
Molecular Weight : 108,14 g/mol
-
Log P : 1,1
-
Fusion Point : -15°C
-
Boiling Point : 205°C
-
Detection Threshold : 1,2 et 1000 ppb (0,0001%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 93°C
Uses
Uses in perfumery :
Benzyl Alcohol is used in majority as a solvent for some ingredients, even if it is rarely the case. It can also be used is some white flowers accords, to bring volatility.
Year of discovery :
Data not available.
Natural availability :
Benzyl Alcohol is found in low quantity in many natural ingredients. It is found for example in Clove Leaf EO, Damask Rose Absolute and Narcissus Absolute.
Isomerism :
Benzyl Alcohol is an isomer of para-Cresol. These two molecules, although close structurally speaking, do not have the same smell at all, much more animalic on para-Cresol part.
Synthesis precursor :
Benzyl Alcohol can be used to synthesize Benzaldehyde, by adding nitric acid to oxydize it. This oxydation can also be carried out with copper-magnesium oxide pumice. Numerous esterifications can be carried out using Benzyl Alcohol, to obtain compounds as Benzyl acetate, Benzyl Salicylate... Eventually, heating this molecule with strong acids or bases may form Dibenzyl Oxide.
Synthesis route :
Two synthetic routes can be used to obtain Benzyl Alcohol. The first one is a hydrolysis of benzyl chloride, by heating this molecule with alkaline and earthy-alkaline hydroxydes and carbonates. Up to 10% Dibenzyl Ether is also resulting from this reaction. A second route is an oxydation of toluene into benzyl hydroperoxyde. Subsequent hydrolysis synthesizes Benzyl Alcohol and Benzaldehyde. A purification can then isolate Benzyl Alcohol.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,45 % 0,14 % 0,34 % 2,5 % 0,64 % 0,17 % 0,34 % 0,057 %1,5 % Cat.5A B C DCat.6 0,64 % 0,17 % 0,34 % 0,057 %1,5 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,68 % 0,68 %0,057 % 2,2 % 2,2 % 8,5 %0,057 % 0,057 %No Restriction Cat.10A BCat.11A BCat.12 2,2 % 8,5 %0,057 % 0,057 %No Restriction
Annexe I :
Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Balsam absolute, Peru | Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms | 8007-00-9 | 0,43 |
| Balsam oil, Peru | Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms | 8007-00-9 | 1,57 |
| Balsam resinoid, Peru | Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms | 8007-00-9 | 0,17 |
| Cinnamon leaf oil | Cinnamomum zeylanicum Blume | 8015-91-6 | 0,1 |
| Cassie absolute | Vachellia farnesiana (L.) Willd. | 8023-82-3 | 2,7 |
| Champaca absolute | Michelia champaca L. | 8006-76-6 | 0,64 |
| Clove bud oil terpenes | Syzygium aromaticum (L.) Merr. & L.M.Perry | 8000-34-8 | 0,01 |
| Jasmine absolute | Jasmium grandifloum L. | 8022-96-6 | 1,08 |
| Jasmine concrete | Jasminum grandiflorum L. | 8022-96-6 | 1,08 |
| Mimosa absolute | Acacia decurrens (Wendl.f.) Willd. | 8031-03-6 | 0,19 |
| Narcissus poeticus absolute | Narcissus poeticus L. | 68917-12-4 | 2,8 |
| Carnation absolute | Dianthus caryophyllus L. | 8021-43-0 | 0,2 |
| Rose oil | Rosa x damascena Mill. | 8007-01-0 | 0,02 |
| Rose absolute | Rosa x damascena Mill. | 90106-38-0 | 0,54 |
| Styrax absolute | Liquidambar styraciflua L. | 8046-19-3 | 2,5 |
| Styrax essence pyrogenated | Liquidambar Styraciflua | 8046-19-3 | 0,15 |
| Styrax oil | Liquidambar styraciflua L. | 8046-19-3 | 0,07 |
| Thyme absolute | Thymus vulgaris L. | 8007-46-3 | 0,3 |
| Tuberose absolute | Poliantes tuberosa L. | 8024-05-3 | 0,5 |
| Tuberose concrete | Poliantes tuberosa L. | 8024-05-3 | 0,5 |
| Violet leaf absolute | Viola odorata L. | 8024-08-6 | 0,1 |
| Ylang ylang oil I | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,09 |
| Ylang ylang oil II | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,09 |
| Ylang ylang oil III | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,04 |
| Ylang, Ylang oil complete | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,25 |
| Ylang, Ylang oil extra | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,24 |
| Hyacinth absolute | Hyacinthus orientalis L. | 8023-94-7 | 40 |
| Jasmine officinale absolute | Jasminum officinale L. | 8024-43-9 | 3 |
| Jasmine sambac absolute | Jasminum sambac (L.) Aiton | 103798-23-6 | 4,2 |
| Jasmine sambac CO2 extract | Jasminum sambac | 103798-23-6 | 4,2 |
| Lavandin grosso absolute | Lavandula angustifolia Mill. x Lavandula latifolia Medik. | 617-009-6 | 0,02 |
| Mimosa concrete | Acacia decurrens Willd. var.dealbata | 98903-76-5 | 0,04 |
| Ylang-ylang absolute | Cananga odorata (Lam.) Hook.f.&Thomson forma genuina | 0,5 | |



