Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Citronellol 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
Citronellol Natural | CT-501 |
Visit website
|
Natural |



|
100 | Cymbopogon winterianus jowitt | Citronella Oil | Indonesia | 50 Kgs |
|
|
Citronellol | 30035053 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 106-22-9
-
EINECS number : 203-375-0
-
FEMA number : 2309
-
FLAVIS number : 02.011
-
JECFA number : 1219
-
Appearance : Colorless liquid
-
Density : 0,855
-
Volatility : Heart
-
Price Range : €
Physico-chemical properties
-
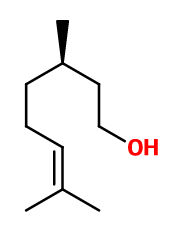
Molecular formula : C10H20O
-
Molecular Weight : 156,27 g/mol
-
Log P : 3,3
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 225°C
-
Detection Threshold : 11 ppb à 2,2 ppm (0,00022%). 40 ppb pour sa forme lévogyre, plus puissante.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 100°C
Uses
Uses in perfumery :
Citronellol is used in reconstitutions of rose and in citrus notes. Often provides an olfactory base for detergents. Used in soaps and home care products for its stability.
Year of discovery :
Data not available.
Natural availability :
Citronellol is present in many plants. It can mainly be extracted from Boronia citriodora (up to 80% of (+) - Citronellol) or Eucalyptus citriodora (from 15 to 20% of (+) - Citronellol). The laevorotatory isomer is more present in Damask Rose EO (and other roses) and Geranium EO. The extracts of all these plants allow to obtain Citronellol in its natural state.
Isomerism :
As already mentioned, the asymmetric carbon of Citronellol imposes a difference between its laevorotatory and dextrorotatory enantiomers. The smell of the laevorotatory isomer is more delicate, powerful and floral. Dihydromyrcenol, with a floral note, and Aldehyde C-10, less floral but more aldehydic and hesperidic, are both constitutional isomers of Citronellol.
Synthesis precursor :
Citronellol is a precursor to the synthesis of many molecules. For example, dehydrogenation of Citronellol provides Citronellal. Moreover, several esters are obtained by reacting various carboxylic acids with Citronellol in the presence of an acid catalyst.
Synthesis route :
The synthesis of Citronellol can be done in several ways. A catalytic hydrogenation of Citronellal allows to obtain it, taking care of the isomery of the Citronellal that is used (in the case of a natural Citronellal, its isomerism is guided by the plant from which it is extracted). A second synthetic route is made from Geraniol and Nerol or Citral by a regioselective hydrogenation. This regioselectivity is guided by catalysis (metallic or aminic) and the conditions of the reaction. The laevorotatory enantiomer of Citronellol can finally be prepared from dextrorotatory alpha-Pinene or beta-Pinene, whose hydrogenation allows to obtain (+)-cis-Pinane. A pyrogenation of this molecule allows to synthesize (+)-3,7-dimethyl-1,6-octadiene. Then, Citronellol is obtained by reaction of this product with triisobutylaluminium or diisobutylaluminium hydride, followed by an air oxidation and a hydrolysis.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 2,2 % 0,67 % 13 % 12 % 3,2 % 3,2 % 3,2 % 3,2 %7,3 % Cat.5A B C DCat.6 3,2 % 3,2 % 3,2 % 3,2 %7,3 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 25 % 25 %1,3 % 24 % 87 % 87 %48 % 48 %No Restriction Cat.10A BCat.11A BCat.12 87 % 87 %48 % 48 %No Restriction
Annexe I :
Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Schinus molle oil | Schinus areira L. | 68917-52-2 | 0,02 |
| Hibawood oil | Thujopsis dolabrata (Thunb. Ex L.f.) Sieb.& Zucc. | 68917-43-1 | 0,38 |
| Citron oil | Citrus medica L. | 68991-25-3 | 0,12 |
| Citronella oil, Ceylon type | Cymbopogon nardus (L.) Rendle | 8000-29-1 | 6 |
| Citronella oil, Java type | Cymbopogon winterianus Jowitt | 8000-29-1 | 11 |
| Spruce oil, Black | Picea mariana (Mill.) Britton | 8008-80-8 | 0,2 |
| Eucalyptus citriodora oil | Corymbia citriodora (Hook.) K.D. Hill & L.A. Johnson | 85203-56-1 | 7,37 |
| Orange flower oil, bitter (neroli and neroli bigarade) | Citrus aurantium L. spp. Amara Link | 8016-38-4 | 0,04 |
| Lemongrass oil, East Indian | Cymbopogon flexuosus (Nees ex Steudel) Will. Watson | 8007-02-1 | 0,25 |
| Lime oil distilled | Citrus aurantifolia (Christm.) Swingle | 8008-26-2 | 0,01 |
| Litsea cubeba oil | Litsea Cubeba(Lour.) Pers. | 68855-99-2 | 0,15 |
| Balm oil | Melissa officinalis L. | 8014-71-9 | 7,49 |
| Niaouli oil | Melaleuca viridiflora Sol. ex Gaertn. | 8014-68-4 | 0,1 |
| Pine, Pinus pumila, extract | Pinus Pumila (Pall) Regel | 0,1 | |
| Rose oil | Rosa x damascena Mill. | 8007-01-0 | 32,5 |
| Rose water stronger | Rosa x centifolia L. | 8007-01-0 | 1,2 |
| Rose absolute | Rosa x damascena Mill. | 90106-38-0 | 4,02 |
| Rose concrete | Rosa x damascena Mill. | 90106-38-0 | 4,7 |
| Thyme oil, red | Thymus vulgaris L. | 8007-46-3 | 0,02 |
| Verbena absolute | Lippia citriodora (L.) Kunth | 8024-12-2 | 0,45 |
| Bucchu absolute, betulina | Agathosma betulina (P.J.Bergius) Pillans | 84649-93-4 | 0,19 |
| Citrus hystrix extract | Citrus hystrix DC | 91771-50-5 | 3 |
| Marjoram oil, Spanish | Thymus mastichina L. | 8016-33-9 | 0,09 |
| Orange flower water absolute | Citrus aurantium L. spp. Amara Link | 8030-28-2 | 0,02 |
| Spruce oil, White | Picea abies (L.) H.Karst. | 91770-69-3 | 0,28 |



