Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Indol - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 120-72-9
-
EINECS number : 204-420-7
-
FEMA number : 2593
-
FLAVIS number : 14.007
-
JECFA number : 1301
-
Appearance : Pale yellow solid
-
Density : 1,22
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
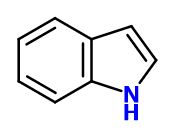
Molecular formula : C8H7N
-
Molecular Weight : 117,15 g/mol
-
Log P : 2,14
-
Fusion Point : 52°C
-
Boiling Point : 254°C
-
Detection Threshold : 0,1831 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 121°C
Uses
Uses in perfumery :
Indole is to be used in reconstructions of jasmine, rose and neroli for example. More generally used to bring an animalic facet to all types of accords.
Year of discovery :
1869
Natural availability :
Indole is present in Grandiflorum Jasmine Absolute, Narcissus Absolute and Orange Blossom Absolute among others, and can be extracted in its natural state. The natural production of Indole, which is the main production method for this molecule, is made by fractional distillation of coal tar, resulting from the pyrolysis of certain woods. The fraction used to recover Indole is the one that is obtained with a temperature between 428 and 500 °F.
Isomerism :
Indole does not have any isomer used in perfumery.
Synthesis precursor :
Indole forms a Schiff base with several aldehydes and ketones such as Citronellal, Dihydrojasmone or Hydroxycitronellal, among others. These Schiff bases are used in perfumery. It can also form several esters in reaction with carboxylic acids.
Synthesis route :
Indole is a compound of the pyrrole family. These molecules can be synthesized in several ways. In the case of Indole, a Pall-Knorr synthesis is the most suitable. This reaction consists in reacting 2- (2-oxo-cyclohex-3,5-dienyl) acetaldehyde with ammonia. Many other synthetic routes exist specifically for this compound, such as the synthesis of Bischler-Möhlau, of Reissert or the Madelung synthesis among others.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment


