Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Acetate de Phenyl ethyle - 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
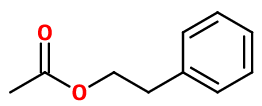
PHENYL ETHYL ACETATE | M_0053300 |
Visit website
|
Naturel | - | - | - | - | - |
General Presentation
-
CAS N° : 103-45-7
-
EINECS number : 203-113-5
-
FEMA number : 2857
-
FLAVIS number : 09.031
-
JECFA number : 989
-
Appearance : Colorless liquid
-
Density : 1,033
-
Volatility : Heart
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C10H12O2
-
Molecular Weight : 164,2 g/mol
-
Log P : 2,27
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 239°C
-
Detection Threshold : 40 à 200 ppb (0,00002%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 105°C
Uses
Uses in perfumery :
Phenyl ethyl acetate is used in addition to phenyl ethyl alcohol, for its even more honeyed and fruity note, in reconstitutions of rose or lilac.
Year of discovery :
Data not available.
Natural availability :
Phenyl ethyl acetate is present in small quantity in several commonly used extracts: Ylang-Ylang Extra EO (and other ylang fractions), Narcissus Absolute, Indian Geranium EO, Champaca Absolute, and in several consumer products such as liquors. It can be extracted in its natural state from the above-mentioned extracts.
Isomerism :
Phenyl Ethyl acetate has several constitutional isomers used in perfumery. These include Styrallyl acetate, from which one of the carbons in the main chain is ramified, and which has a much more fruity and green smell, reminiscent of rhubarb. Finally, there is Benzyl Propionate, whose esterified chain is longer than the chain linked to the aromatic cycle, to get a more fruity smell of pear and jasmine.
Synthesis precursor :
Phenyl Ethyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Phenyl Ethyl acetate can be synthesized by an esterification reaction between Phenyl Ethyl Alcohol and acetic acid, in the presence of a catalyst such as concentrated sulfuric acid, in small quantity in the reaction medium. The yield of this reaction can be improved by using acetic anhydride or chloroacetic acid instead of acetic acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment


