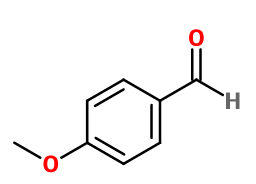
Anisic Aldehyde
Naturelle - Synthétique
Balsamic Ambery > Almondy > Coumarinic > Anisic > Vanillic
Crédits photo: ScenTree SAS
Other names :
Para-Anisaldehyde ; 4-methoxybenzaldehyde ; Anise aldehyde ; Aubepine ; Crategine ; Formyl anisol
Volatility :
Base
Uses in perfumery :
Anisic Aldehyde is used in lilac and mimosa accords, for a sweet vegetable, aromatic and anisic note.
Natural availability :
Anisic Aldehyde is present in Star Anise EO, Anise EO and Ceylon Cinnamon EO (and other origins), from which it can be extracted.
Year of discovery :
Anisaldehyde was discovered in 1895 by scientist Auguste Cahours, by oxydation of an anise oil.
Other comments :
Anisic Aldehyde was used for the first time in perfumery in Après l'Ondée, by Guerlain, in 1906.
Anisic aldehyde is more vanillic than Anisyl Alcohol and less fruity than Anisyl acetate.
Anisic aldehyde is more vanillic than Anisyl Alcohol and less fruity than Anisyl acetate.
Price Range :
€€
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time

Crédits photo: ScenTree SAS
- Molecular formula :
- C8H8O2
- Molecular Weight :
- 136,15 g/mol
- Density :
- 1,119
- Flash Point :
- 116°C
- Fusion Point :
- -5°C
- Appearance :
- Colorless liquid
- Log P :
- 1,65
- Boiling Point :
- 248°C
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
Its synthesis is made by an oxidation from para-Cresol methyl ether, in the presence of manganese dioxide, air or peroxidised compounds (the catalysis of these reactions is metallic). Other routes exist, such as the two-step synthesis from para-Cresol, via 4-hydroxybenzaldehyde, which is then oxidized. A last synthesis route is an electrochemical oxidation in the presence of an aliphatic alcohol. The intermediate product of this route is acetal, corresponding to Anisic Aldehyde.
Synthesis precursor :
Anisic Aldehyde is the precursor for the synthesis of Anisic Alcohol by hydrogenation and Anisic Acid by oxidation. It can also react with Methyl Anthranilate or Indole to form a Schiff base.
Isomerism :
The isomer used here is para-Anisic Aldehyde. The ortho and meta isomers have a relatively similar smell.
Methyl Benzoate and Phenylacetic Acid are constitutional isomers of Anisaldehyde. Their smell is however very different.
- EINECS number :
- 204-602-6
- FEMA number :
- 2670
- JECFA number :
- 878
- FLAVIS number :
- 05.015
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,043 % 0,08 % 0,022 % 0,21 % 0,11 % 0,022 % 0,032 % 0,0072 % 0,011 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,022 % 0,022 % 0,0072 % 0,065 % 0,065 % 0,21 % 0,0072 % 0,0072 % 4,9 % - Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 51
- Comments :
- This Standard is set due to the phototoxic effects of Methyl β-naphthyl ketone. For more detailed information on the application of this Standard, please refer to the note on phototoxic ingredients in chapter 1 of the Guidance for the use of IFRA Standards.
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,23 % 0,080 % 0,14 % 1,4 % 0,38 % 0,047 % 0,14 % 0,031 % 0,42 % 0,19 % 0,031 %
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


