Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Acetate de Linalyle - 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
Linalyl Acetate | 30034993 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Linalyl Acetate BMBcert™ | 30786718 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 115-95-7
-
EINECS number : 204-116-4
-
FEMA number : 2636
-
FLAVIS number : 09.013
-
JECFA number : 359
-
Appearance : Colorless liquid
-
Density : 0,901
-
Volatility : Heart
-
Price Range : €
Physico-chemical properties
-
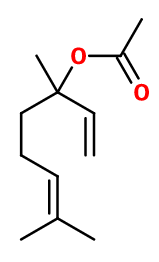
Molecular formula : C12H20O2
-
Molecular Weight : 196,29 g/mol
-
Log P : 3,93
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 220°C
-
Detection Threshold : 1 ppm (0,0001%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 94°C
Uses
Uses in perfumery :
Linalyl acetate is used in bergamot and lavender accords. Brings a marked freshness in chypre (accompanied by patchouli, oakmoss and cistus among others) and oriental perfumes (accompanied by balsamic and vanilla notes).
Year of discovery :
Data not available.
Natural availability :
Linalyl acetate is the major component of Bergamot EO, as it is present at about 30 to 40%. Therefore, it can be isolated to obtain natural Linalyl acetate. In addition, acetylation of Lavender EO and Petitgrain Bigarade EO enriches Linalyl acetate, as they contain a high level of Linalool. It is called hemi-synthetic Linalyl acetate. Linalyl acetate can also be removed by fractional distillation to produce it in its natural state.
Isomerism :
Linalyl acetate used in perfumery is a racemic mixture of its two (R) and (S) enantiomers. Both have a close smell. Geranyl acetate, Neryl acetate, Terpenyl acetate and Isobornyl acetate are isomers of Linalyl acetate. However, the first two are reminiscent of pear and rose, Isobornyl acetate is reminiscent of pine, and Terpenyl acetate also has a smell of Bergamot EO, although more fruity-sweet.
Synthesis precursor :
Linalyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Linalyl acetate is obtained synthetically by an esterification reaction between Linalool and acetic acid or acetic anhydride in an acid medium. Linalool is synthesized in two stages from Myrcene. Linalyl acetate can also be synthesized in two stages from Myrcene, by adding hydrochloric acid, catalysed by copper chloride II. The second step consists in reacting acetic acid with the intermediate product obtained in the presence of sodium acetate and dichloromethane.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment


