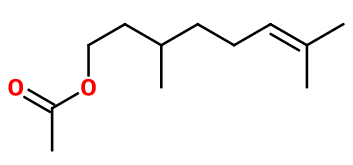
Citronellyl acetate
Naturelle - Synthétique
Floral > Rosy > Green Fruits > Aquatic
Crédits photo: ScenTree SAS
Other names :
Citronellyl acetate ; 3,7-dimethyloct-6-enyl acetate ; acetate de 3,7-dimethyloct-6-enyl ; Citronellyl ethanoate ; Acetic acid citronellyl ester ; Citronellol acetate ; 3,7-dimethyloct-6-enol acetate ; 3,7-dimethyloct-6-enyl ethanoate ; 3,7-dimethyloct-6-enol ethanoate
Volatility :
Head/Heart
Uses in perfumery :
Citronellyl acetate is used for a fruity note in rose, lily of the valley and lavender accords for example.
Natural availability :
Citronellyl acetate is naturally present in Lemongrass EO, Geranium EO and Rose de Mai Absolute, and therefore is extractable in order to obtain natural Citronellyl acetate.
Year of discovery :
Data not available.
Other comments :
Citronellyl acetate has a less green apple note than Geranyl acetate or Neryl acetate. May contain traces of Citronellol and/or Geraniol and therefore a check must be made as it becomes a possible allergen.
Price Range :
€€
Stability :
acetates may form acetic acid through time

Crédits photo: ScenTree SAS
- Molecular formula :
- C12H22O2
- Molecular Weight :
- 198,31 g/mol
- Density :
- 0,891
- Flash Point :
- 93°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Colorless liquid
- Log P :
- 4,22
- Boiling Point :
- 240°C
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
Citronellyl acetate can be synthesized by an esterification reaction between Citronellol and acetic acid or acetic anhydride, in an acid medium. It can also be synthesized from 3,7-dimethylocta-1,6-diene, obtained naturally by pyrolysis of alpha-Pinene. This synthesis is made in three steps: a Markovnikov addition reaction using hydrochloric acid, a Kharasch reaction, also called anti-Markovnikov reaction, with hydrobromic acid, followed by an acetolysis reaction using sodium ethanoate.
Synthesis precursor :
Citronellyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Isomerism :
The asymmetric carbon of Citronellol gives it two different smells if its enantiomers are separated: the (R)-(+)-Citronellyl acetate is fruity and rosy, while the (S)-(-)-Citronellyl acetate is more aldehydic, dirty and lemony. In perfumery, those two enantiomers can be used separately. In most cases, a mixture of the two is used.
Menthanyl acetate, Verdox® and Vertenex® are constitutional isomers of Citronellyl acetate. However, Menthanyl acetate is much more reminiscent of Bergamot EO, and Verdox® and Vertenex® are woodier.
- EINECS number :
- 205-775-0
- FEMA number :
- 2311
- JECFA number :
- 57
- FLAVIS number :
- 09.012
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 51
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,49 % 0,15 % 2.0 % 2,7 % 0.70 % 0,82 % 2,4 % 0,23 % 5,4 % 0,41 % 0,23 %
This ingredient is not restricted for the 49th amendment
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


